Effective, FDA-indicated, non-invasive treatment for depression in Arkansas.
What is TMS?
TMS stands for Transcranial Magnetic Stimulation. It is a non-invasive neuromodulation technique using short magnetic pulses over the scalp inducing electrical currents in the neurons of the cortex modulation brain function to treat psychiatric diseases, more specifically, depression. The use of the first TMS device was in 1985 with early studies demonstrating robust improvements in depression symptoms (George et al., 1995). After more than 20 years, rTMS is considered an acceptable intervention for depression.
How does TMS work?
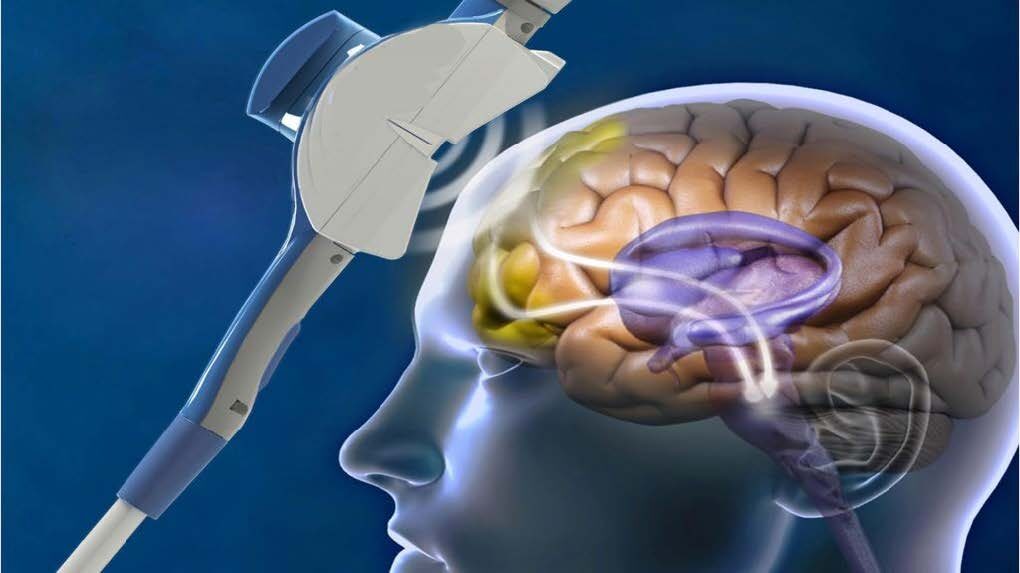
Patients undergoing TMS treatment sit in a dentist-like chair in the doctor’s office with an electromagnet pressed to the left side of their head, dorsolateral prefrontal cortex. The machine produces a pulsed magnetic filed, similar to that used in M.R.I.’s, which creates a small electrical current in the surface of the brain that “resets” the patient’s mood regulatory system.
TMS treatment has been shown to produce changes in the activity of neurons in the regions of the brain involved in mood regulation, such as the prefrontal cortex. As each magnetic pulse passes through the skull and into the brain, this induces brief activity of brain cells underlying the treatment coil. This is like physical therapy for the neurons, improving level of activity/function in areas that have not been functioning as well–often resulting in relief from symptoms of depression.
What is the treatment process?
Sessions
TMS therapy involves a series of treatment sessions. Treatment sessions are approximately 20 minutes each, and administered 5 days a week. A typical course of TMS treatment is 4 to 6 weeks. However, this can vary depending on an individual’s response to treatment. Early symptom improvement is highly predictive of response and guides treatment.
Non-invasive
TMS does not require any sedation or general anesthesia, so it is not necessary to go to a hospital to receive treatment. Patients are fully awake and aware during the treatment and the patient is allowed to read or watch TV. There is no “recovery period,” so patients can drive home afterwards and return to their normal activity.
Does NeuroStar/ TMS work?
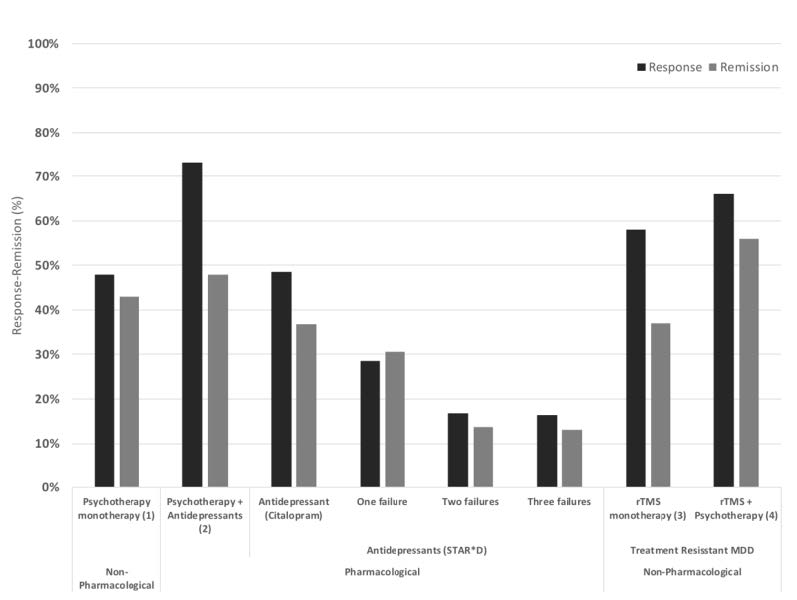
TMS has received a Level A status, meaning definite efficacy/highest level of scientific evidence, and is FDA indicated since 2008 for the treatment of depression and is readily covered by insurance companies. When comparing to second, third, and fourth trial of medication, TMS has a greater response and remission rate.
Does Neurostar / TMS Hurt?
The most common side effect with NeuroStar is that there may be mild to moderate pain or discomfort at the treatment site. For most patients this subsides within the first week of treatment.
Other Considerations
There are considerations when thinking about medication trials vs TMS option, based on the issues we run into with medication:
- remission rates
- weight gain
- medication interactions
- sexual dysfunction
- liver enzyme system impact
- platelet/bleeding issues
- diabetes impact
- sedation/sleep effects
TMS Arkansas is here for you
Our team of specialists seek to provide safe, tolerable, and well researched treatment options for you.
Let us be your guides in the personalized treatment of your depression.
Let us be your support in your individual process of recovery.
Let us be your helping hand to living the life you want–with joy, happiness, and passion unique to you.
Watch the video below and visit our Testimonials page to learn more about how NeuroStar TMS Therapy could help you overcome symptoms of depression.
